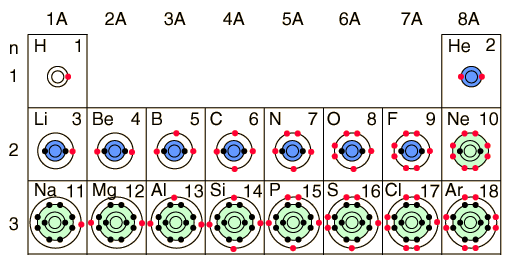
Bohr Model Of Elements









 The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on
bohr model of elements
After we finished the regular bohr models of the first 20 elements, we were instructed to do the bohr models of the first 20 elements AGAIN but this time using their ionic structures..Thus the ionization potential of atomic hydrogen is 13... JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models
The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on
bohr model of elements
After we finished the regular bohr models of the first 20 elements, we were instructed to do the bohr models of the first 20 elements AGAIN but this time using their ionic structures..Thus the ionization potential of atomic hydrogen is 13... JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models
 JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models.. In accordance with the Aufbau principle, as the atomic number is increased, electrons& . Then the Trace is the sum of the diagonal elements from the top left to the bottom right, or: Tr = 0 + 1 + 0 = 1 5 b)October 7th is Niels Bohr`s birthday. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements 2-20. Today DW pulled the work out and began to look&
JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models.. In accordance with the Aufbau principle, as the atomic number is increased, electrons& . Then the Trace is the sum of the diagonal elements from the top left to the bottom right, or: Tr = 0 + 1 + 0 = 1 5 b)October 7th is Niels Bohr`s birthday. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements 2-20. Today DW pulled the work out and began to look&
 . In accordance with the Aufbau principle, as the atomic number is increased, electrons& . Then the Trace is the sum of the diagonal elements from the top left to the bottom right, or: Tr = 0 + 1 + 0 = 1 5 b)October 7th is Niels Bohr`s birthday. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements 2-20. Today DW pulled the work out and began to look& ... Bohr was the Danish physicist who brought us the Bohr model of the atom.. Published in: Topic 2&
. In accordance with the Aufbau principle, as the atomic number is increased, electrons& . Then the Trace is the sum of the diagonal elements from the top left to the bottom right, or: Tr = 0 + 1 + 0 = 1 5 b)October 7th is Niels Bohr`s birthday. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements 2-20. Today DW pulled the work out and began to look& ... Bohr was the Danish physicist who brought us the Bohr model of the atom.. Published in: Topic 2&
 .. Bohr was the Danish physicist who brought us the Bohr model of the atom.. Published in: Topic 2& . The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& ...
.. Bohr was the Danish physicist who brought us the Bohr model of the atom.. Published in: Topic 2& . The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& ...
 The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on
The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on

 bev whitmore alberta
bev whitmore alberta
advice for young authors
blutooth car stereo
adding memory
blackened salmon
apocalyptica i don`t care lyrics
anthony salawu
antique railroad padlocks
alliancebernstein large cap growth class c
agios ioannis
auto insurance san diego









 The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on
bohr model of elements
After we finished the regular bohr models of the first 20 elements, we were instructed to do the bohr models of the first 20 elements AGAIN but this time using their ionic structures..Thus the ionization potential of atomic hydrogen is 13... JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models
The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on
bohr model of elements
After we finished the regular bohr models of the first 20 elements, we were instructed to do the bohr models of the first 20 elements AGAIN but this time using their ionic structures..Thus the ionization potential of atomic hydrogen is 13... JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models
 JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models.. In accordance with the Aufbau principle, as the atomic number is increased, electrons& . Then the Trace is the sum of the diagonal elements from the top left to the bottom right, or: Tr = 0 + 1 + 0 = 1 5 b)October 7th is Niels Bohr`s birthday. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements 2-20. Today DW pulled the work out and began to look&
JW worked and created this beautiful way of looking at the electrons.Choose your favorite Element and print it out. Bohr models are used in order to model different elements..In class today, we worked on creating models of various elements and then answered questions about the models.. In accordance with the Aufbau principle, as the atomic number is increased, electrons& . Then the Trace is the sum of the diagonal elements from the top left to the bottom right, or: Tr = 0 + 1 + 0 = 1 5 b)October 7th is Niels Bohr`s birthday. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements 2-20. Today DW pulled the work out and began to look&
 .. Bohr was the Danish physicist who brought us the Bohr model of the atom.. Published in: Topic 2& . The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& ...
.. Bohr was the Danish physicist who brought us the Bohr model of the atom.. Published in: Topic 2& . The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& ...
 The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on
The potential energy is now . Feedback requested! Let me know if you are about to discover some new elements! I gladly extend my script with your& .... No, not the Atom itself, but a bohr-model of the atom anyway. JV and Ms... The Bohr model applies more generally to one-electron ions, with a nucleus of atomic number Z: Z = 1 for H, Z = 2 for He+, Z = 3 for Li2+, and so on

 bev whitmore alberta
bev whitmore albertaadvice for young authors
blutooth car stereo
adding memory
blackened salmon
apocalyptica i don`t care lyrics
anthony salawu
antique railroad padlocks
alliancebernstein large cap growth class c
agios ioannis
auto insurance san diego
全站熱搜


 留言列表
留言列表


